Procedure Library
Sponsored Projects
Policies & Compliance
- Research Conduct
- Combatting Human Trafficking
- Federal Disclosure Requirements
- Research Handbook
- 1. Roles & Responsibilities
- 2. Standards for Conduct of Research
- 3. Overview of Sponsored Projects Administration
- 4. Funding Sources & Opportunities
- 5. Proposal Development
- 6. Budget Development
- 7. Procedures for the Submission of Proposals
- 8. Award Acceptance
- 9. Award Management
- 10. Research Related Regulations, Policies & Procedures
- 11. Other Conduct of Research Issues
- 12. Acronyms & Definitions
- 13. Glossary
- Procedure Library
- Regulations Library
Document Summary Sheet (DSS) Creation
Purpose
The Document Summary Sheet (DSS), generated through the eProposal system in CIS, is the official starting point for any sponsored project. e-Proposal enables the electronic preparation, review, and tracking of proposed research activities.
The DSS gathers key information about the proposed project and documents the required institutional approvals.
After all approvals are obtained, the DSS is integrated into PeopleSoft and its data is transferred to eAward. If the proposal is funded - or if a preliminary project is needed - departments may then initiate requests (such as a preliminary project setup or a new award setup). These requests establish the foundation for the financial project(s) in PeopleSoft and the Management Reports system. Data entered in PeopleSoft for proposals and awards is also used for institutional reporting.
Applicability
A new DSS must be created whenever Investigators or administrators submit a sponsored project proposal, including letters of intent or pre-proposals, that require University authorization, institutional approval, or OSP involvement. This includes:
- A new application, whether to a prime sponsor or a pass-through entity
- A resubmission of a previously unfunded proposal
- A new competing segment of an award (e.g., renewal, competing continuation)
- An existing award that will be relinquished and transferred to a new institution when the new institution subcontract back to the University of Utah
- Other non-routine or exceptional situations
When applicable, use the Current UU Project Number field on the Proposal Information page in eProposal to list the previous project number(s), if applicable, to link the previous and new submissions:
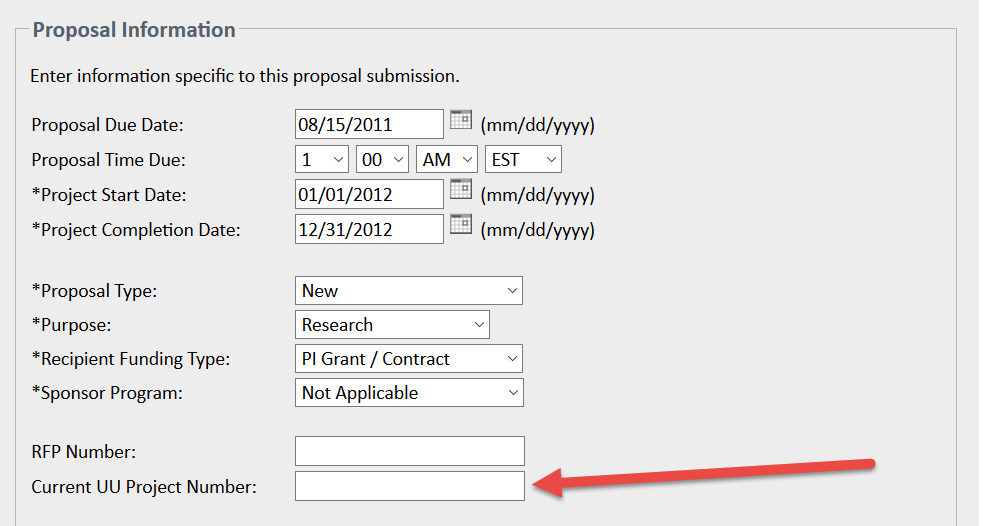
Note: NSF and NIH supplements generally do not require a new DSS, even though they often involve formal submissions. Supplements are generally processed under the same DSS as the parent award, but exceptions may occur - for example, if the supplement is issued under a different Document ID or award number.
Instructions
- Refer to the Obtain Approvals section of the Grant Life Cycle for detailed guidance on completing the DSS.
Related Policies and Procedures
- Grant Life Cycle > Submit Your Proposal > Obtain Approvals
- Grant Life Cycle > Submit Your Proposal > Understand Submission Processes
- Research Handbook > Procedures for the Submission of Proposals > Document Summary Sheet
Be notified of page updates
Procedure Library Feedback
Do you have comments or suggestions for this procedure?
