NIH Data Management & Sharing Policy
Upcoming NIH Requirement Change (Effective for Due Dates On or After May 25, 2026).
NIH has released Notice NOT-OD-26-046, updating required elements of the Data Management and Sharing Plan and introducing
a new simplified DMS Plan format.
Applicants must use the new format for applications due on/after May 25, 2026. Additional
guidance and updates to follow.
2023 DMSP Summary
NIH currently requires grants with $500k per year or more (directs) to provide a brief explanation of how and when data resulting from the grant would be shared.
ALL grant applications or renewals that generate Scientific Data, with due dates on or after January 25, 2023, must now include a robust and detailed plan for how PI's will manage and share data during the project period. This includes information on data storage, access policies/procedures, preservation, metadata standards, distribution approaches, and more.
PI's must provide this information in a data management and sharing plan (DMSP). The approved plan will become part of the award terms and conditions.
Proposal budgets may include requests for estimated funds needed for data management and sharing activities. These should include all allowable costs for data management & sharing for all data types.
As applicable, PI's will need to collaborate closely with the IRB to ensure that the sharing of data pertaining to human subjects is consistent between the DMSP and informed consent.
Scientific Data as Defined by NIH
"Scientific data is the recorded factual material commonly accepted in the scientific community as of sufficient quality to validate and replicate research findings, regardless of whether the data are used to support scholarly publications."
Use this decision tool to determine what sharing policies apply to your researcH
Office of Research Integrity & Compliance (ORIC) Resources
- Document: Guidance on the NIH DMSP (12/9/2022)
NIH Resources
- Policy for Data Management & Sharing (NOT-OD-21-013)
- Updated Elements of an NIH Data Management & Sharing Plan
(NOT-OD-26-046) - Data Management & Sharing Policy Overview
- Toolkit for NIH Data Management & Sharing Policy
- NIH Genomic Data Sharing Policy
- NIH Model Organisms Policy
- NIH Research Tools Policy
- FAQ for the NIH Policy for Data Management and Sharing
- Elements of an NIH DMSP
- Allowable Costs for DMS
- Selecting a Repository
- Informed Consent for Secondary Research with Data and Biospecimens
First Steps
- Determine your personal timeline. If you have an active NIH award going up for renewal with receipt date of January 2023, or if you are planning to submit an NIH proposal this year, then developing a DMSP should be a high priority, especially if you are working with external collaborators as it may take time to set up appropriate data procedures/agreements.
- Read through this webpage to familiarize yourself with the changes and with the policy itself (including the supplements)
- Familiarize yourself with the FAIR principles (Wilkinson et. al, 2016). The FAIR (findable, accessible, interoperable, reusable) data principles are the guiding principles the NIH has used in creating the new policy.
- Assess your own project and data management practices relative to the policy (see the NIH-provided supplements below), especially around documenting existing practices and developing new ones to address the increased emphasis on data sharing and administrative oversight.
- Review campus data services (e.g., computing, storage, consulting) and assess whether they will meet your needs. Also consider costs you may need to budget for such as labor for data cleaning and documentation (see the NIH-provided supplement on allowable costs).
As applicable, all new proposals must include a DMSP. After your funding is awarded, execute your ICO-approved plan and document that work in your RPPR to comply.
Step 1: Review data management and sharing best practices prior to creating your plan
- The NIH maintains a list of repositories. There is also a UU libguide on NIH Data Management & Sharing Policy
- Sample data management plans
Step 2:
(a) Establish what data needs to be managed and by whom.
(b) Establish what data needs to be shared under the policy. The rule of thumb is to make the data "as open as possible, as closed as necessary".
(c) Document the following in your DMSP:
-
Of the data being collected, which datasets would be subject to additional privacy controls? Who will manage such controls? Who will have access? Refer to the Information Security Office and Patient Privacy as applicable.
-
If you have a Data Use Agreement, ensure that the agreement allows for de-identified data sharing and/or sharing of derived datasets, if possible.
-
If your research involves human subjects, ensure that consent forms contain appropriate language that allows de-identified data sharing. Contact the Institutional Review Board (IRB)
-
If your research involves Indigenous populations, ensure that the appropriate leaders/groups have approved your plan for collection or use.
Step 3: Write the DMSP.
![]() We suggest using the DMPTool to create your plan (sign in with your uNID). Begin by selecting the "Create a Plan"
button. It includes a template with guidance on what to write in each section of the
DMSP. Completed plans can be downloaded and shared.
We suggest using the DMPTool to create your plan (sign in with your uNID). Begin by selecting the "Create a Plan"
button. It includes a template with guidance on what to write in each section of the
DMSP. Completed plans can be downloaded and shared.
- Writing a Data Management and Sharing Plan
- Elements to Include in a Data Management and Sharing Plan
Alternatively, NIH has an optional DMS Plan form available and Sample data management plans. You may also be interested in this Framework for Creating a Data Management Plan.
Considerations for specialized datasets, data privacy, and human subjects protections:
- Applications subject to both the DMS Policy and the Genomic Data Sharing (GDS) Policy will submit a single Plan. See NOT-OD-22-198 for details and other considerations.
- Human subjects participant data protections: Informed Consent, De-identification, Certificate of Confidentiality
- Any restrictions on sharing: Regulatory restrictions (e.g. HIPAA, GDPR), Tribal laws, policies and Tribal sovereignty, ethical considerations
If your research requires IRB approval, the IRB may ask for information contained in your DMSP. Therefore, it is strongly recommended to draft your DMSP prior to seeking IRB approval.
Step 4: Attach the DMS plan in the Other Plans section within Forms H as part of your NIH application.
-
DSMP PDF MUST be attached in the new “Other Plans” attachment point; cannot be uploaded in “Resource Sharing Plans” attachment point
- Resource Sharing Plan can be used for other requirements
-
-
Still could be used for Model Organisms sharing
-
(Genomic Data Sharing plans should now be in the DMSP)
-
Step 5: Proposal budgets may include requests for estimated funds needed for data management and sharing activities. These should include all allowable costs for data management & sharing for all data types.
- Are you required to deposit in a certain repository? What is the Fee?
- Are there storage needs above any repository size limit and what is the impact?
- Will you need to determine long-term costs & negotiate pre-payment?
- Is your NIH budget modular or non-modular?
- Will you need dedicated staff time for any data management functions? Will you need to hire new staff?
- Do you have any subrecipients and will they be responsible/need to budget for data management and sharing?
- Some services provided by the University are free of charge while others are not. While free services do not need to be included in the budget, please consider contact the managing office prior to including them in your DMSP (e.g., repositories).
Guidelines for budgeting for data management & SHaring
- Label as Data Management and Sharing Costs:
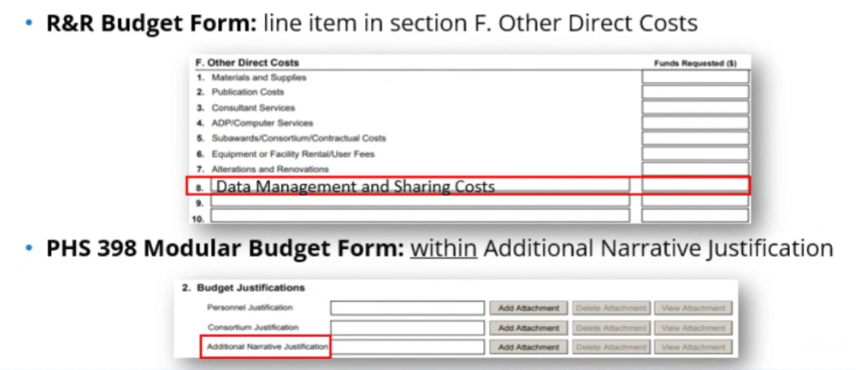
- A brief summary must be provided in the budget justification, even if there are no costs related to DMSP.
- Must be incurred before end of the project period. For example, costs for long-term
data preservation must be budgeted for in the proposal and paid before the end of
the grant. You may find the NIHM Data Archive (NDA) cost estimation worksheet or the publication Forecasting Costs for Preserving, Archiving, and Promoting Access to Biomedical Data useful.
Step 6: Peer Review/Post Award
-
NIH staff may have peer reviewers evaluate plan if DMSP is integral to the project and a DMSP plan is required by the FOA
-
If DMSP is deemed not sufficient, additional info will be requested during JIT (PI should talk to PO)
-
NIH will monitor compliance with each DMSP over the course of the funding period during regular reporting intervals (e.g., at the time of annual Research Performance Progress Reports (RPPRs)). Noncompliance with Plans may result in the NIH ICO adding special Terms and Conditions of Award or terminating the award. If PI's are not compliant with their DMSP at the end of the award, noncompliance may be factored into future funding decisions.
Step 1: If you have a grant already underway, review this webpage and compare your existing data practices to the new requirements as soon as possible.
Step 2: Identify gaps in your existing plans and practices. The new policy requires much more robust data management and sharing plans. Your updated plan must address data sharing, which may not be a component of your existing work plan. Other areas that may need attention are: data use agreements, carving out time for data preparation (eg., de-identification), documentation, and upload into a data repository.
Step 3: To draft the plan, review the Guidance for New Proposals above.
Learning
The Network of the National Library of Medicine (NNLM) is promoting a free DMPTool webinar on creating comprehensive data management plans using the tool. This webinar will guide attendees through data management plan basics, creating a DMPTool profile, and exploring available templates and planning resources. This training is well timed to help researchers prepare for the new NIH Data Management and Sharing Policy effective for all NIH funded research this coming January 2023.
View recording of DMPTool training
Webinars:
|
Webinar I: Understanding the New NIH Data Management and Sharing (DMS) Policy |
Webinar II: Diving Deeper into the new NIH Data Management and Sharing Policy |


